Synthesis of Asymmetric Pyrazoline Derivatives from Phenylthiophenechalcones; DFT Mechanistic Study
FigureS1.
The geometrical structure of 3aii and 3bii.
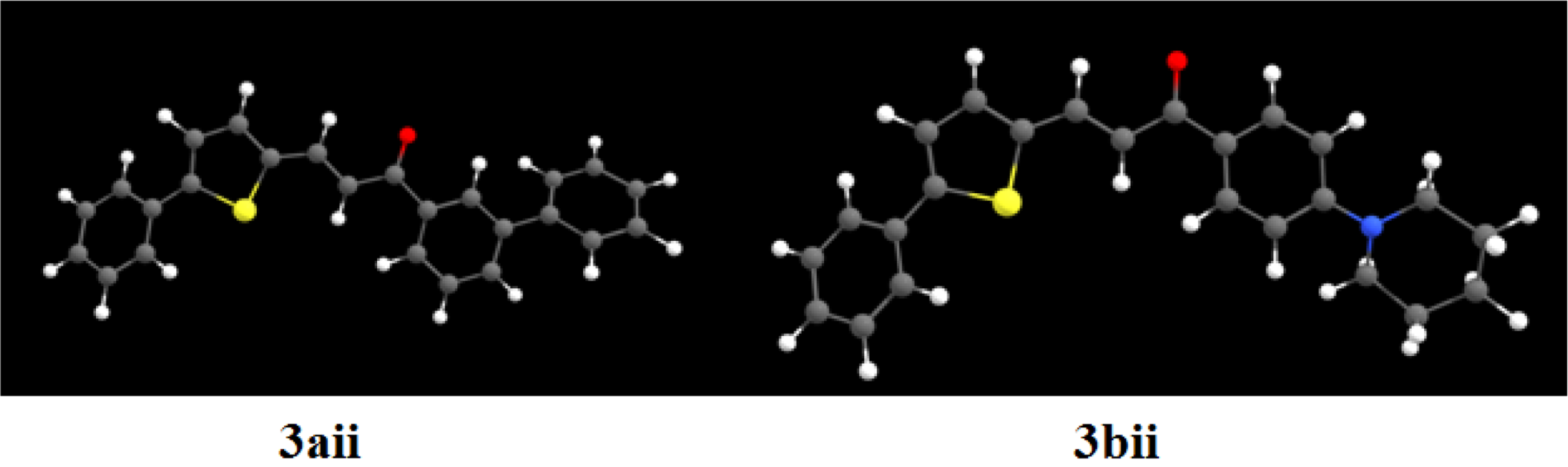
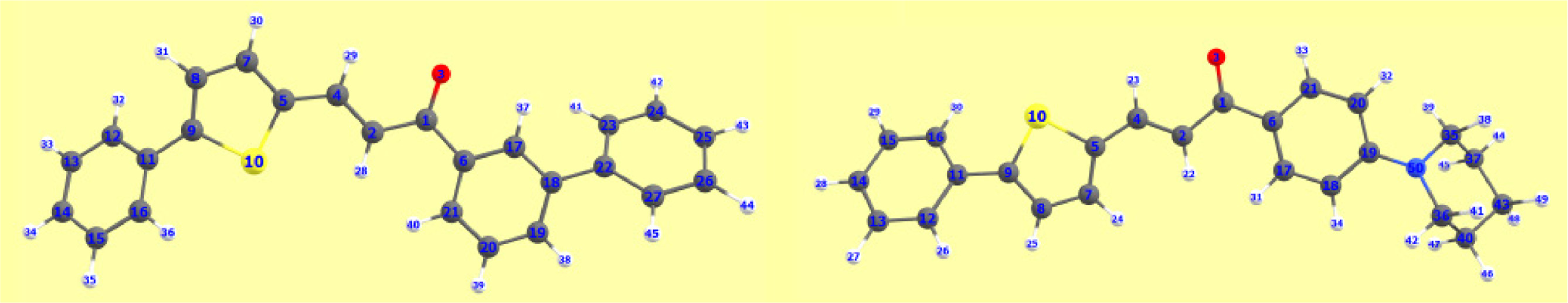
FigureS2.
APT charges of 3aii and 3bii.
FigureS3.
The geometrical structures of the most stable conformers of pyrazole derivative 4a, b.
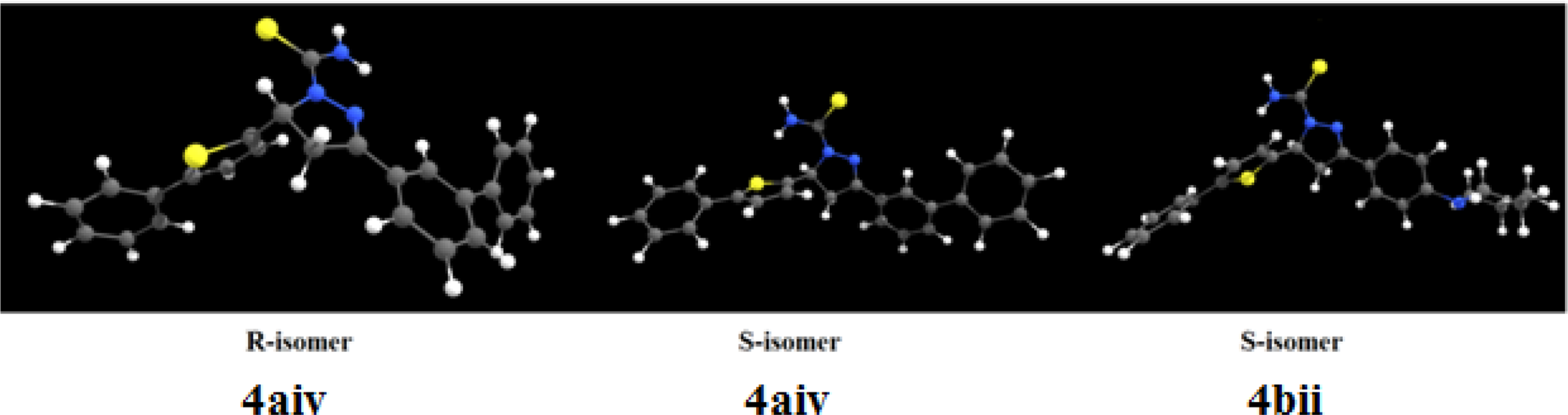
FigureS4.
Experimental Ultraviolet visible spectrum of the compounds 3a, b and 4a, b.
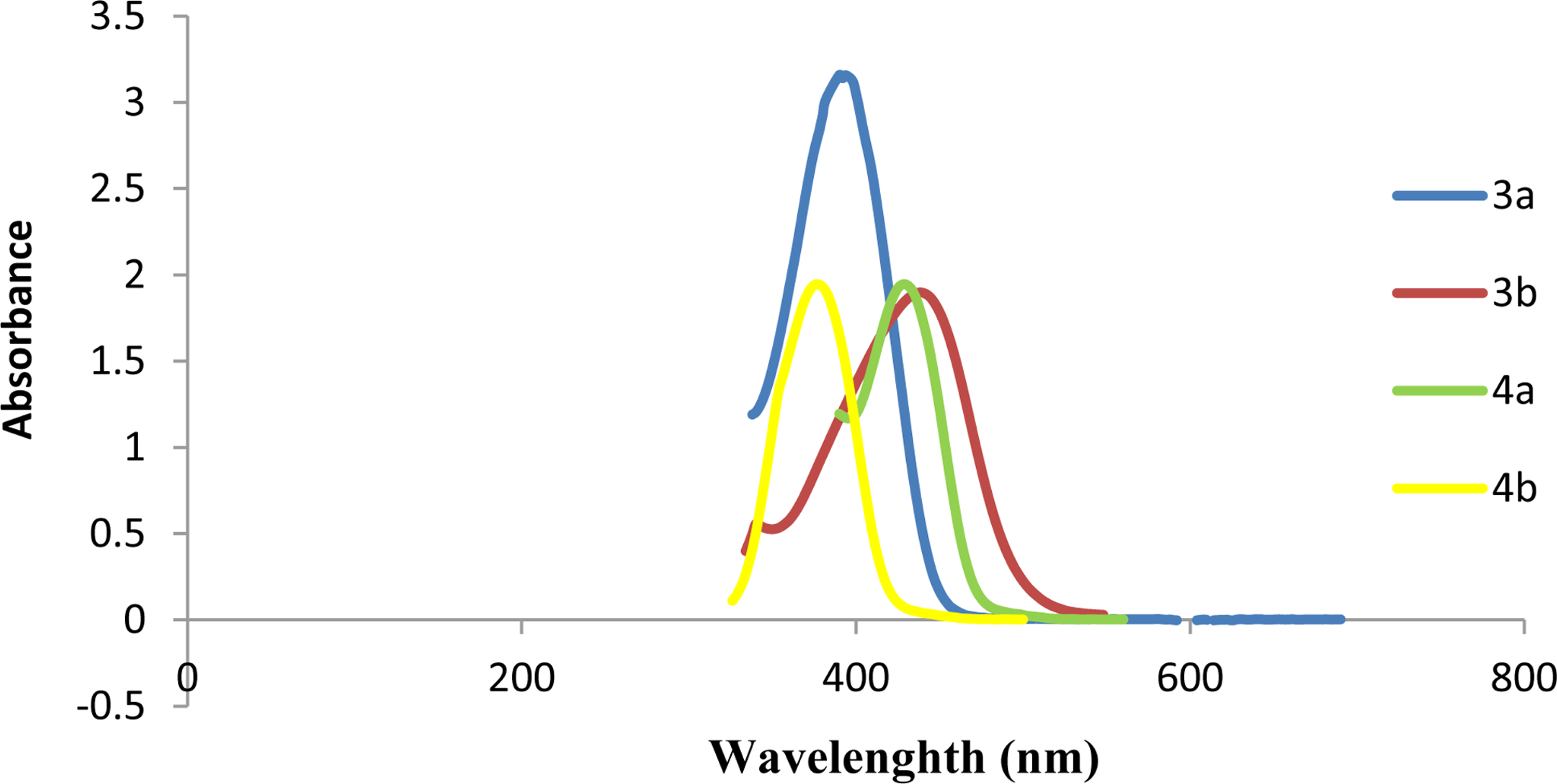
FigureS5.
1H NMR of 1-(biphenyl-4-yl)-3-(5-phenylthiophen-2-yl) prop-2-en-1-one (3a).
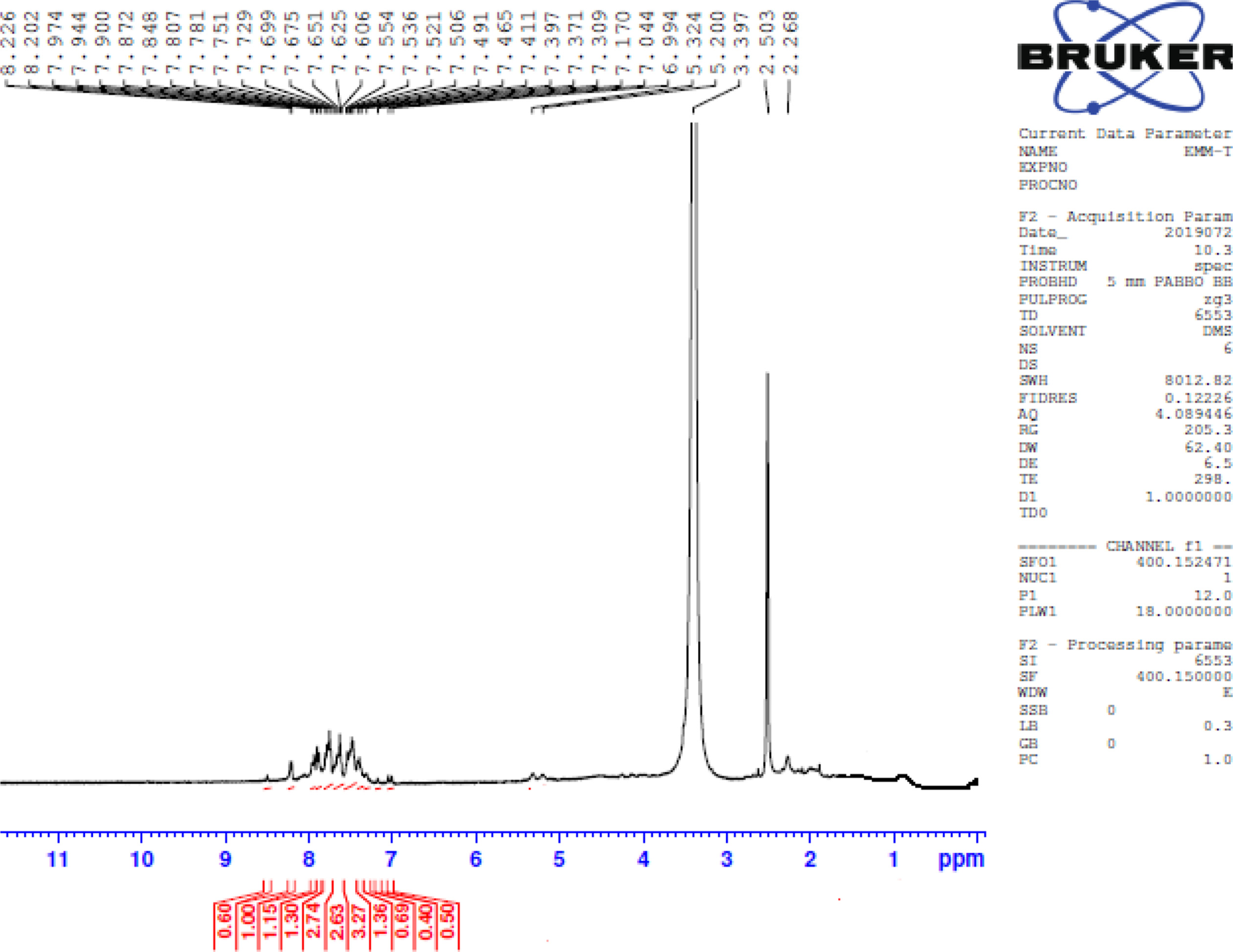
FigureS6.
1H NMR of 3-(5-phenylthiophen-2-yl)-1-(4-(piperidin-1-yl) phenyl) prop-2-en-1-one (3b).
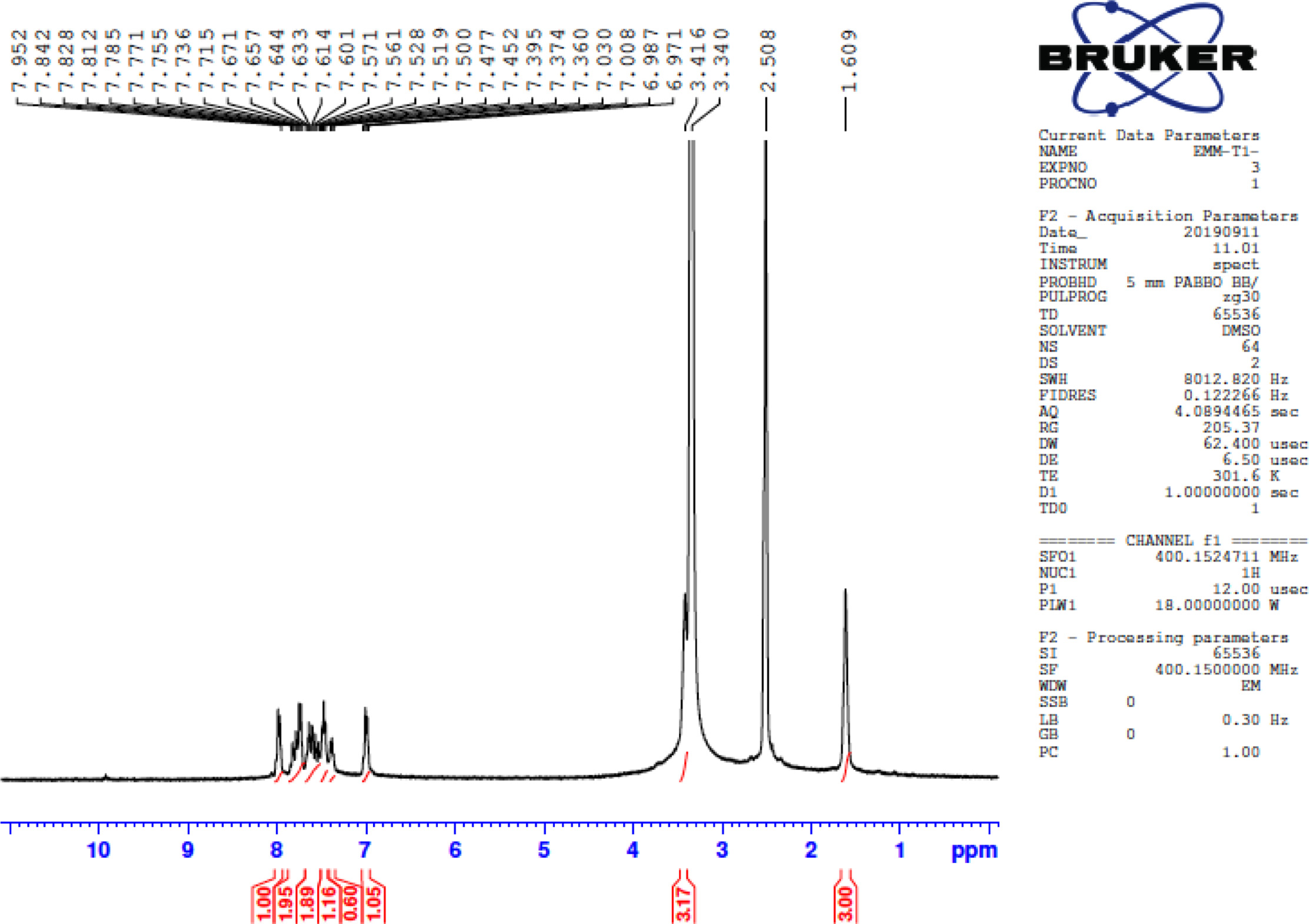
FigureS7.
1H NMR of 5-(biphenyl-4-yl)-3-(5-phenylthiophen-2-yl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (4a).
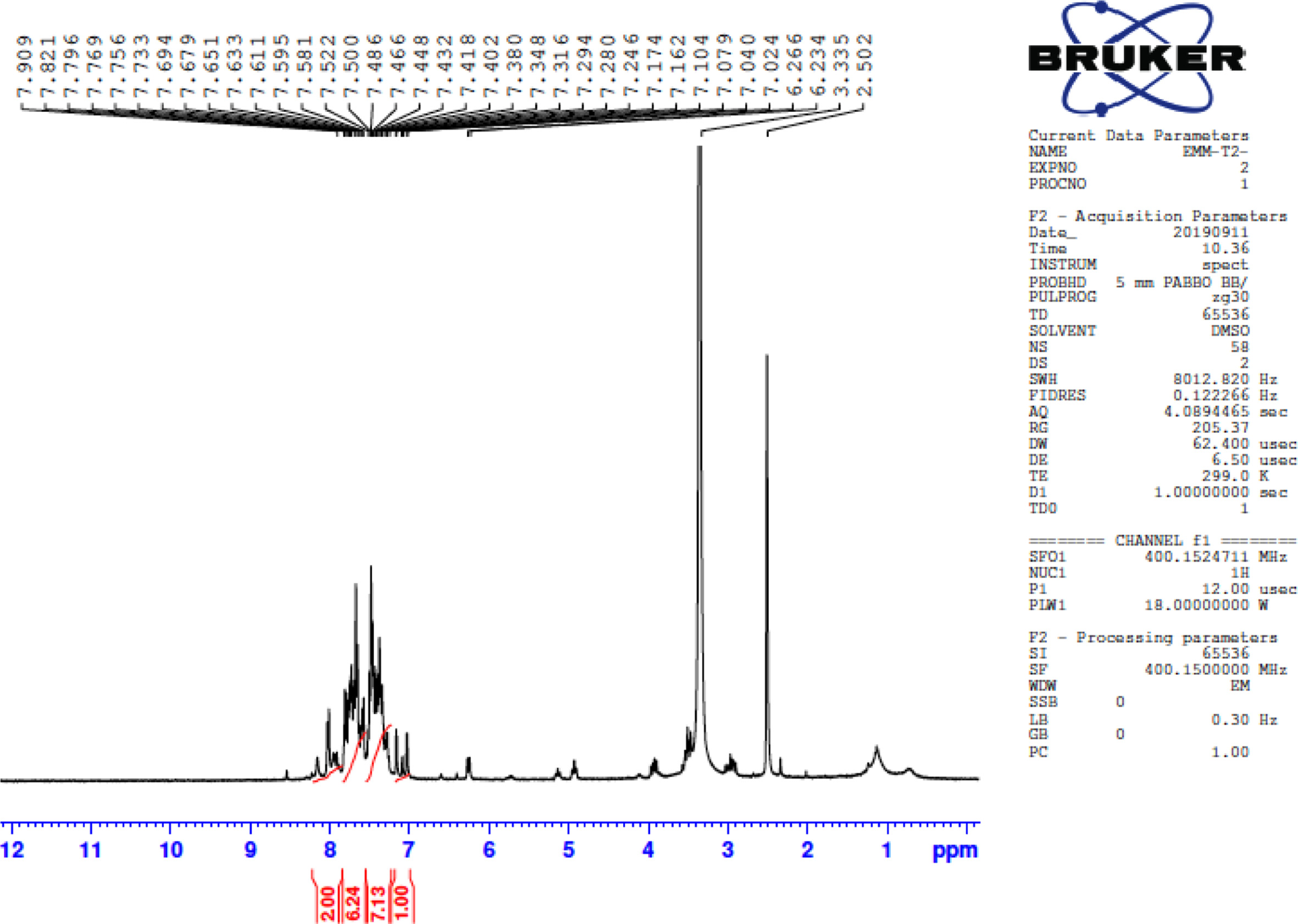
FigureS8.
1H NMR of 3-(5-phenylthiophen-2-yl)-5-(4-(piperidin-1-yl) phenyl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (4b).
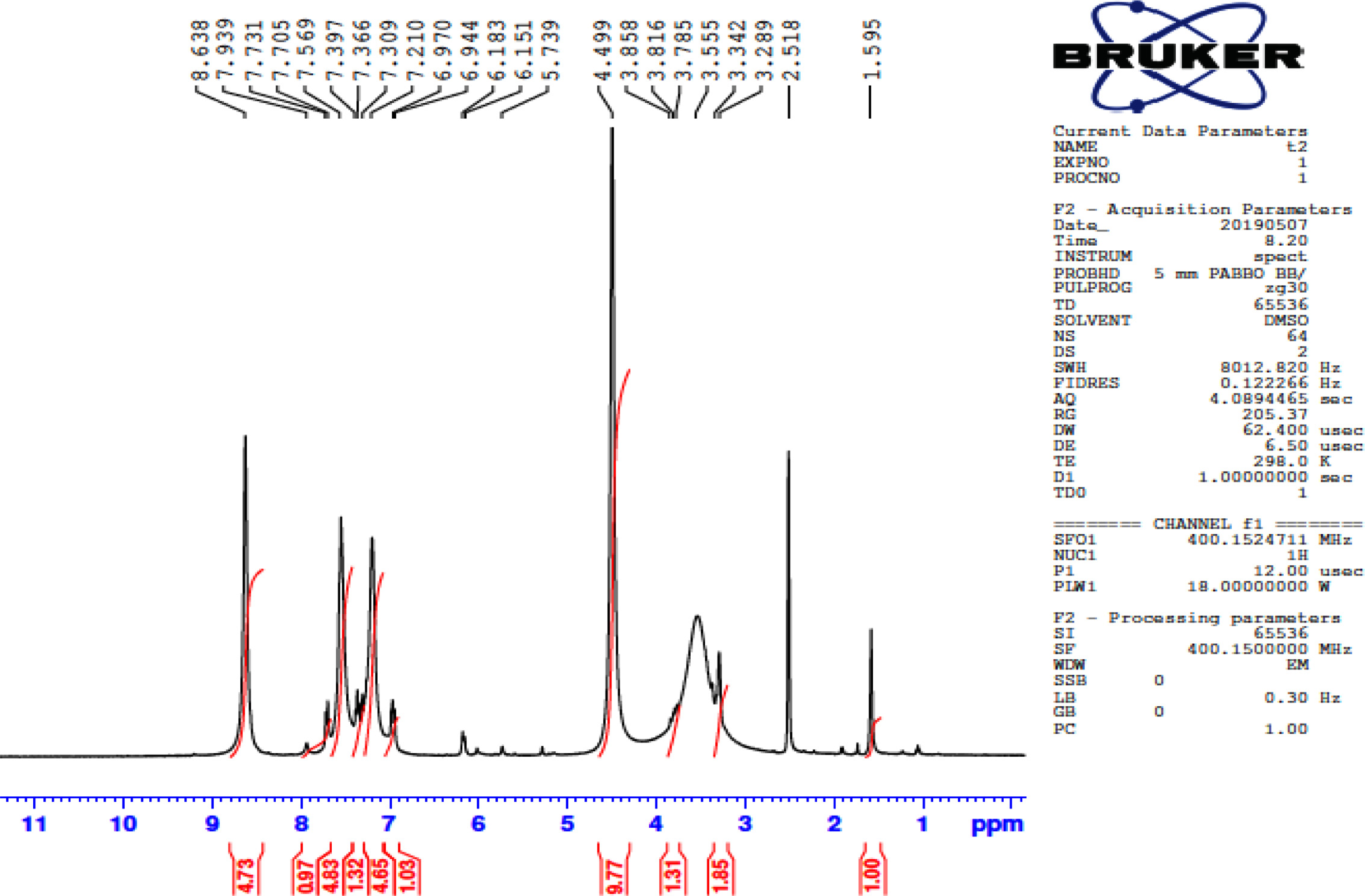
FigureS9.
13C NMR of 3-(5-phenylthiophen-2-yl)-1-(4-(piperidin-1-yl) phenyl) prop-2-en-1-one (3b).
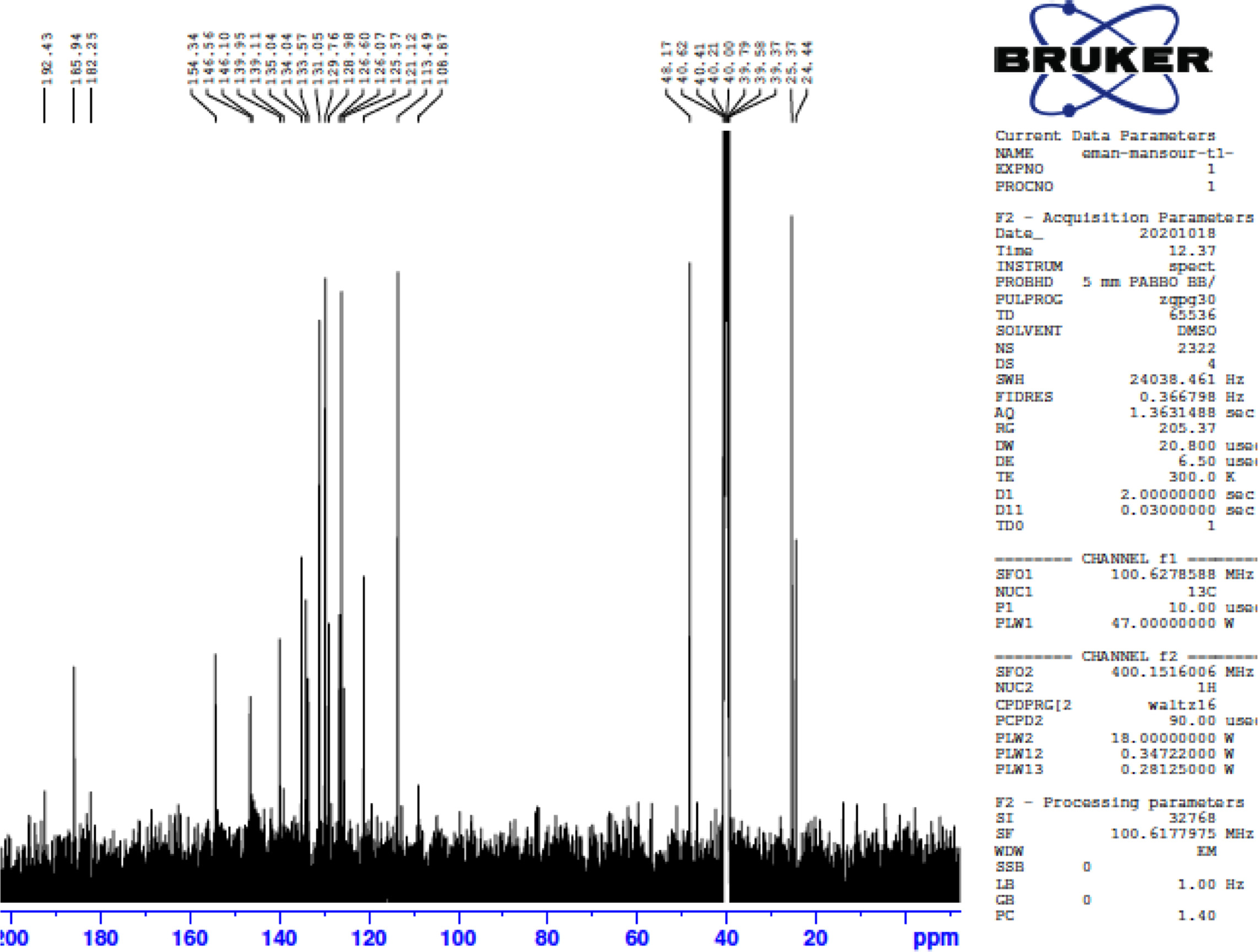
FigureS10.
13C NMR of 5-(biphenyl-4-yl)-3-(5-phenylthiophen-2-yl)-4,5-dihydro-1H-pyrazole-1-carbothioamide (4a).
TableS1.
Thermal parameters (Hartree/Particle) of 3a (i-iv) and 3b (i, ii)
|
Parameter
|
3ai
|
3aii
|
3aiii
|
3aiv
|
3bi
|
3bii
|
|
Ecorr |
0.354267
|
0.354236
|
0.354051
|
0.354080
|
0.415871
|
0.412301
|
|
ZPVE
|
-1436.525705
|
-1436.525925
|
-1436.522804
|
-1436.522877
|
-1455.855579
|
-1456.106969
|
|
Etot |
-1436.503716
|
-1436.503922
|
-1436.500689
|
-1436.500777
|
-1455.832396
|
-1456.083566
|
|
H
|
-1436.502771
|
-1436.502978
|
-1436.499745
|
-1436.499833
|
-1455.831452
|
-1456.082622
|
|
G
|
-1436.581677
|
-1436.581686
|
-1436.579320
|
-1436.579128
|
-1455.912580
|
-1456.163920
|
|
ΔH of 3a |
0.12989
|
0.00000
|
2.02874
|
1.97352
|
|
|
|
ΔH of 3b |
|
|
|
|
157.71811
|
0.00000
|
TableS2.
APT charges of 3aii and 3bii
|
Atoms
|
3aii
|
3bii
|
|
1 C
|
1.542513
|
1.694828
|
|
2 C
|
-0.817673
|
-0.767780
|
|
3 O
|
-0.884223
|
-0.926469
|
|
4 C
|
0.716468
|
0.628943
|
|
5 C
|
-0.364727
|
-0.173074
|
|
6 C
|
-0.317217
|
-0.811183
|
|
7 C
|
0.251588
|
0.103723
|
|
8 C
|
-0.214063
|
-0.263964
|
|
9 C
|
0.156180
|
0.146624
|
|
10 S
|
-0.089358
|
-0.017232
|
|
11 C
|
0.080135
|
0.153855
|
|
12 C or N
|
-0.021715
|
-0.059755
|
|
13 C
|
0.006669
|
0.010964
|
|
14 C
|
-0.045686
|
-0.071076
|
|
15 C
|
-0.005776
|
0.008667
|
|
16 C
|
-0.037949
|
-0.047131
|
|
17 C
|
0.079282
|
0.319468
|
|
18 C
|
0.109220
|
-0.404756
|
|
19 C
|
-0.038199
|
0.989711
|
|
20 C
|
-0.054161
|
-0.367081
|
|
21 C
|
-0.036071
|
0.430065
|
|
22 C
|
0.102400
|
0.305929
|
|
23 C
|
-0.042429
|
0.280923
|
|
24 C
|
0.010784
|
0.003678
|
|
25 C
|
-0.045645
|
0.007774
|
|
26 C
|
0.001180
|
0.021064
|
|
27 C
|
-0.041530
|
-1.196716
|
TableS3.
Thermal parameters (Hartree/Particle) of R and S of 4a (i-iv) and S of 4b (i, ii)
|
Parameter
|
4ai R
|
4ai S
|
4aii R
|
4aii S
|
4aiii R
|
4aiii S
|
4aiv R
|
4aiv S
|
4bi S
|
4bii S
|
|
Ecorr |
0.4082
|
0.4097
|
0.4097
|
0.4098
|
0.4130
|
0.4098
|
0.4103
|
0.4097
|
0.4677
|
0.4678
|
|
ZPVE
|
-1963.5430
|
-1963.5789
|
-1963.5792
|
-1963.5789
|
-1963.2749
|
-1963.5790
|
-1963.5914
|
-1963.5792
|
-1983.1615
|
-1983.1619
|
|
Etot |
-1963.5209
|
-1963.5530
|
-1963.5533
|
-1963.5530
|
-1963.2491
|
-1963.5531
|
-1963.5655
|
-1963.5533
|
-1983.1343
|
-1983.1347
|
|
H
|
-1963.5200
|
-1963.5521
|
-1963.5523
|
-1963.5521
|
-1963.2481
|
-1963.5522
|
-1963.5646
|
-1963.5523
|
-1983.1333
|
-1983.1337
|
|
G
|
-1963.5950
|
-1963.6397
|
-1963.6404
|
-1963.6393
|
-1963.3355
|
-1963.6398
|
-1963.6523
|
-1963.6404
|
-1983.2240
|
-1983.2239
|
TableS4.
Selected structure parameters of optimized geometry of stable S isomers of 4aiv and 4bii calculated by DFT B3LYP/6-311G method
|
Structural Parameters
|
4aiv S
|
4aiv R
|
4bii S
|
|
Ra) |
|
|
|
|
C1-C2
|
1.46308
|
1.46000
|
1.46316
|
|
C2-C3
|
1.51963
|
1.52322
|
1.51954
|
|
C3-C4
|
1.55778
|
1.54662
|
1.55774
|
|
C4-N5
|
1.49955
|
1.49757
|
1.49971
|
|
N5-N6
|
1.40125
|
1.40918
|
1.40131
|
|
C2-N6
|
1.30099
|
1.29839
|
1.30098
|
|
C4-C7
|
1.50580
|
1.51094
|
1.50589
|
|
C10-C12
|
1.46448
|
1.46528
|
|
|
C22-C26
|
1.48797
|
1.48775
|
|
|
Aa) |
|
|
|
|
C1-C2-C3
|
124.958
|
125.356
|
124.960
|
|
C1-C2-N6
|
121.549
|
122.030
|
121.549
|
|
N5-C4-C7
|
111.293
|
33.768
|
111.280
|
|
C4-C7-C8
|
129.384
|
129.180
|
129.366
|
|
C4-C7-S11
|
119.875
|
120.799
|
119.893
|
|
Da) |
|
|
|
|
N5-C4-C7-S11
|
37.162
|
31.658
|
159.907
|
|
C3-C2-C1-C21
|
1.291
|
175.345
|
179.300
|
|
C9-C10-C12-C17
|
150.400
|
151.431
|
150.401
|