Journal Information
Journal ID (publisher-id): chemical
Title: Journal of the Korean Chemical Society
Translated Title (ko): 대한화학회지
ISSN (print): 1017-2548
ISSN (electronic): 2234-8530
Publisher: Korean Chemical Society대한화학회
Kinetic study is an important method for mechanistic investigation in organic chemistry. Value evidence such as activation parameters, the effect of solvent or substituents, kinetic solvent isotope effects, and evaluating of differences between related structures could provide valuable information about reaction mechanism.
The Grunwald-Winstein equation1 (eq. 1) is a useful tool for correlating the solvent dependence of rates of solvolysis. The sensitivity (m) can be determined by studying the rates of solvolysis reaction of a standard substrate. However, Fainberg and Winstein2 found that the plots for some substrates showed a scattering of plots in binary solvent systems due to the solvent nucleophilicity.
Nucleophilic solvent assistance can be defined as an electron donation from solvent to the developing positive dipole of a reacting C-X bond and an electron acceptance by the solvent of the leaving group (X), Scheme 1.
Kevill and coworks3 established a solvent nucleophilicity scale based on the solvolysis of S-methyldibenzo-thiophenium ion. They introduced a new term governed by the solvent nucleophilicity. The extended Grunwald-Winstein equation can be expressed as equation 2. In equation 2, k and ko represent the rate constants of the solvolyses of a substrate RX in a given solvent and in the standard solvent (80% ethanol), respectively; l is the sensitivity of the solvolysis to changes in solvent nucleophilicity (NT)4 ; m is the sensitivity of the solvolysis to changes in solvent ionizing power (Yx, for a leaving group X)5; c is a constant (residual). A considerable recent effort
has been to exerted study the extent to which the solvent nucleophilicity and the solvent ionizing power scales, developed for a substitution at carbon, can be applied to the substitution reactions taking place at a hetero atom.4b
The nucleophilic substitution reaction of benzenesulfonyl chloride (C6H5SO2Cl) has been extensively studied.6 In this reaction, chloride is displaced by a nucleophile at the sulfur atom (S) on sulfonyl group. Both SN17 and SN28 mechanism have been reported.
In limiting SN2 mechanism, the nucleophile approaches by donation of its electron pair begins to form a bond to sulfur while the sulfur-leaving group bond is breaking (Scheme 1). The reaction is complected in a single step and both the nucleophile and the substrate take part in the transition state with the rate is second-order.9
On the other hand, the reaction could be a limiting SN1 mechanism for the solvolysis of benzenesulfonyl chloride, Scheme 2, where substrate is ionized without a nucleophilic assistance.9
The purpose of this study is to gain a further understanding of the mechanism of sulfonyl transfer. We carried out kinetic investigations of the solvolyses of 4-(chlorosulfonyl)biphenyl (C6H5C6H4SO2Cl, 1) in a variety of pure and binary solvents at 55.0 °C, Eq. (3).
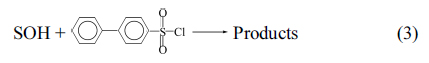
Solvents were purified as previously described.10 4-(chlorosulfonyl)biphenyl (1, 98%) was used as received. The substrate did not react with the pure acetonitrile within the stock solution.
The electrical conductance of a solution depends strongly on the concentration and identity of any ion present. The following nucleophilic displacement reaction in solvent has been studied through conductance measurements. The fact that the reactants are neutral and the products are charged, so the conductance increase as the reaction proceeds. Pseudo-first-order rates (kobs) were followed conductimetrically and the kobs values were obtained by the curve fitting method.10b The rate (k) values reported are the averages of more than triplicate runs and were reproducible to within ±3%.
Solvolysis rate constants for 4-(chlorosulfonyl)biphenyl in ethanol, methanol, and binary mixtures of water with ethanol,
methanol, acetone, and 2,2,2-trifluroethanol (TFE) and the binary mixtures of TFE with ethanol are summarized in Table 1 together with the
Rates were observed to increase in binary aqueous mixture solvents with increasing water content (Table 1). These results insisted that the solvolysis of 1 is dominated by a bimolecular reaction mechanism.11
aUnless otherwise indicated, a 1.0 mol dm−3 solution of the substrate in the indicated solvent, containing 0.1% CH3CN. bOn a volume-volume content at 25.0 °C, other component is water. cValues from ref. 4. dValues from ref. 5. eValues of k [=1.23×10−3s−1] in deuterated methanol (CH3OD), corresponding to kCH3OH/kCH3OD value of 1.26. fSolvent prepared on a weight-weight basis at 25.0 °C, other component is water. gTFE-ethanol mixtures.
The dielectric constants of TFE (ε = 26.7) is about three times smaller than and H2O (ε = 80.0). The dielectric constant of EtOH (ε = 24.3) is quite similar to that for TFE.12 The solvent nucleophilicity exhibit a concerted increase in TFE-EtOH mixtures with increasing EtOH content. Although the ionizing power decreases of the similarity in dielectric constants, this effect is counter balanced and swamped out by the change in solvent nucleophilicity. These results showed normal behavior since ionizing power increases as water fraction increases.
The enthalpies (ΔH≠) and the entropies (ΔS≠) of activation for solvolysis of 1 are determined in four solvents in Table 2. Relatively small ΔH≠ (12.9~15.2 kcal·mol−1) and a large negative ΔS≠ (−28.1~−36.0 cal·mol−1·K−1) values are consistent with the proposed bimolecular reactions.10b
aA 1.0 mol dm−3 solution of the substrate in the indicated solvent, also containing 0.1% CH3CN. bThe activation parameters are accompanied by the standard error. cOn a volume-volume content at 25.0 ℃, other component is water.
The application of the extended Grunwald-Winstein equation (2) to the solvolyses of 1 led to only moderate correlation with dispersal for different binary mixtures. For all kinds of solvents, values obtained were 0.60 ± 0.09 for l, 0.47 ± 0.03 for m; the standard error of the estimate was 0.04; the correlation coefficient (R) value was 0.940 (Fig. 1). The sensitivity values, l and m, are reported in Table 3, along with the corresponding parameters obtained in the analyses of previously studied substrates, to compare them with literature values for related substrates.
In general, for an SN1 reaction without nucleophilic assistance, l value would be zero and m value would be close to unity while l value would be near unity and m value would be near 0.5 for a conventional SN2 mechanism.10 We compare l value (= 0.60) for the solvolysis of 1 with reported of the solvolysis of 9-fluorenyl chloroformate16 (l = 0.95), dimethoxybenzenesulfonyl chloride15 (l = 0.93), N,N-dimethyl sulfamoyl chloride15 (l = 0.92), and benzenesulfonyl chloride15 (l = 1.10) which are believed to be normal SN2 mechanism in Table 3. The l value of 0.60 for the solvolyses of 1 is smaller than those for the solvolyses proceed through a SN2 mechanism (l = 0.92~1.10). This l value (= 0.60) is similar to those previously reported for the solvolyses of benzylsulfonyl chloride (2)16 proposed as SN2 mechanism with some SN1 reaction pathway. Therefore, this similarity suggest that the solvolysis of 1 proceed through an SN2 mechanism involving an attack by solvent at sulfur atom in substrate with some character of SN1 mechanism.
The l/m values from the extended Grunwald-Winstein equation could be a useful mechanistic criteria; l/m values of 1.4 to 2.5 for bimolecular mechanism; below 0.5 for an ionization pathway10 (Table 3). For solvolysis of 1, the l/m value was 1.3 which is within the similar range of the proposed bimolecular pathway.
A kinetic solvent isotope effect (kCH3OH/kCH3OD) of 1.26 ± 0.04 at 55.0 °C is observed. In general, for the SN2 reaction, KSIEs are in the range of values from 1.58 to 2.31, whereas for an SN1 reaction without nucleophilic assistance, KSIEs are close to unity.16 The lower KSIE value (1.26) for 1 compare to the normal SN2 mechanism suggests a dissociative SN2 mechanism with some SN1 reaction.
The solvolysis rate constants of 4-(chlorosulfonyl)biphenyl (1) in 35 different solvents are well correlated with the extended Grunwald-Winstein equation, using the NT solvent nucleophilicity scale and the YCl solvent ionizing power scale, with sensitivity values of 0.60 and 0.47 for l and m, respectively. The activation enthalpies (∆H≠) were 12.9 to 15.2 kcal·mol−1, the activation entropies (∆S≠) were −28.1 to −36.0 cal·mol−1·K−1, and the kinetic solvent isotope effect was 1.26. Based on these results, we suggest that the solvolysis of 1 has a dissociative SN2 mechanism with some character of SN1 reaction.
[(a)] D. N. Kevill M. Charton Advances in Quantitative Structure-Property RelationshipsJAI PressGreenwich, CT1996481115 [(b)] N. D. Kevill M. J. D’Souza Collect. Czech. Chem. Commun.1999641790 [CrossRef]
[(a)] O. Rogne J. Chem. Soc, B.1969663 [CrossRef] [(b)] B. C. Lee I. Lee J. Korean Chem. Soc.198024342
[(a)] S. R. Kim H. J. Choi J. K. Park I. S. Koo H. J. Koh Bull. Korean Chem. Soc.20143551 [CrossRef] [(b)] C. K. Ingold Structure and Mechanism in Organic Chemistry2d ed.Cornell University PressIthaca, N. Y.1969427457
[(a)] S. H. Lee C. J. Rhu J. B. Kyong D. K. Kim D. N. Kevill Bull. Korean Chem. Soc.200728657 [CrossRef] [(b)] H. J. Koh S. J. Kang D. N. Kevill Bull. Korean Chem. Soc.200930383 [CrossRef] [(c)] M. H. Seong S. H. Choi Y. W. Lee J. B. Kyong D. K. Kim Bull. Korean Chem. Soc.2009303408 [(d)] M. H. Seong J. B. Kyong Y. H. Lee D. N. Kevill Int. J. Mol. Sci.200910929 [CrossRef]
H. J. Koh S. J. Kang C. J. J. Kim Bull. Korean Chem. Soc.200930378 [CrossRef]
J. B. Kyong B. C. Park C. B. Kim D. N. Kevill J. Org. Chem.2000658051 [CrossRef]
M. J. D’Souza D. N. Reed K. J. Erdman J. B. Kyong D. N. Kevill Int. J. Mol. Sci.200910862 [CrossRef]
H. J. Koh S. J. Kang Bull. Korean Chem. Soc.2011323799 [CrossRef]