Electrochemical Determination of As(III) at Nanoporous Gold Electrodes with Controlled Surface Area
FigureS1.
(a) Cyclic voltammograms of NPG electrodes according to reaction time in 0.1 M H2SO4 at a scan rate of 50 mV s-1. (b) Dependence of Rf value of NPG electrodes upon the reaction time. NPG electrodes prepared by anodization in 0.1 M phosphate
buffers (pH 8) containing 1 M KCl. (c) SEM image of typical NPG layers prepared by anodization.
FigureS2.
(a) and (b) SWV response on NPG electrodes with different surface area in 3 μM As(III) + 1.0 M H2SO4. (c) Comparison of blank SWV responses of NPG electrodes in 1.0 M H2SO4 in the absence of As(III).
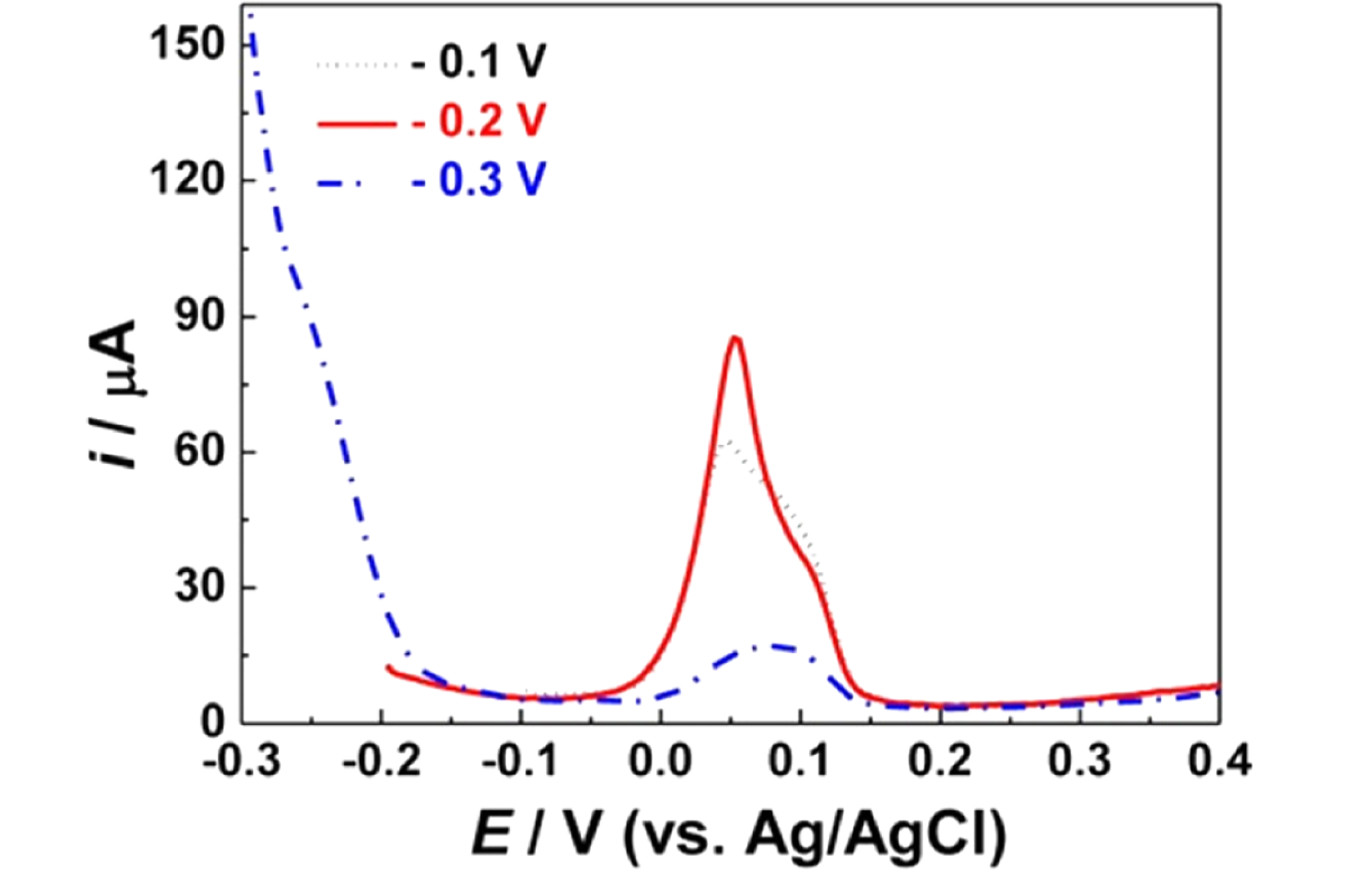
FigureS3.
SWV obtained on NPG (50 s) electrodes in 1 M HCl containing 5 μM As(III) at various deposition potentials. Predeposition time
= 150 s.
FigureS4.
SWVs obtained on a flat Au electrodes in 1 M HCl containing various concentrations of As(III) at different pre-deposition
times.
TableS1.
Dependence upon the deposition time of sensitivity on flat Au electrode and NPG (50 s)
|
Deposition Time (s)
|
Electrode
|
Sensitivity (μA/μM)
|
NPG (50 s)/Flat Au sensitivity
|
|
50
|
Flat Au
|
1.12
|
1.79
|
|
NPG (50 s)
|
2.01
|
|
150
|
Flat Au
|
2.60
|
3.16
|
|
NPG (50 s)
|
8.21
|
|
400
|
Flat Au
|
1.96
|
11.3
|
|
NPG (50 s)
|
22.2
|
FigureS5.
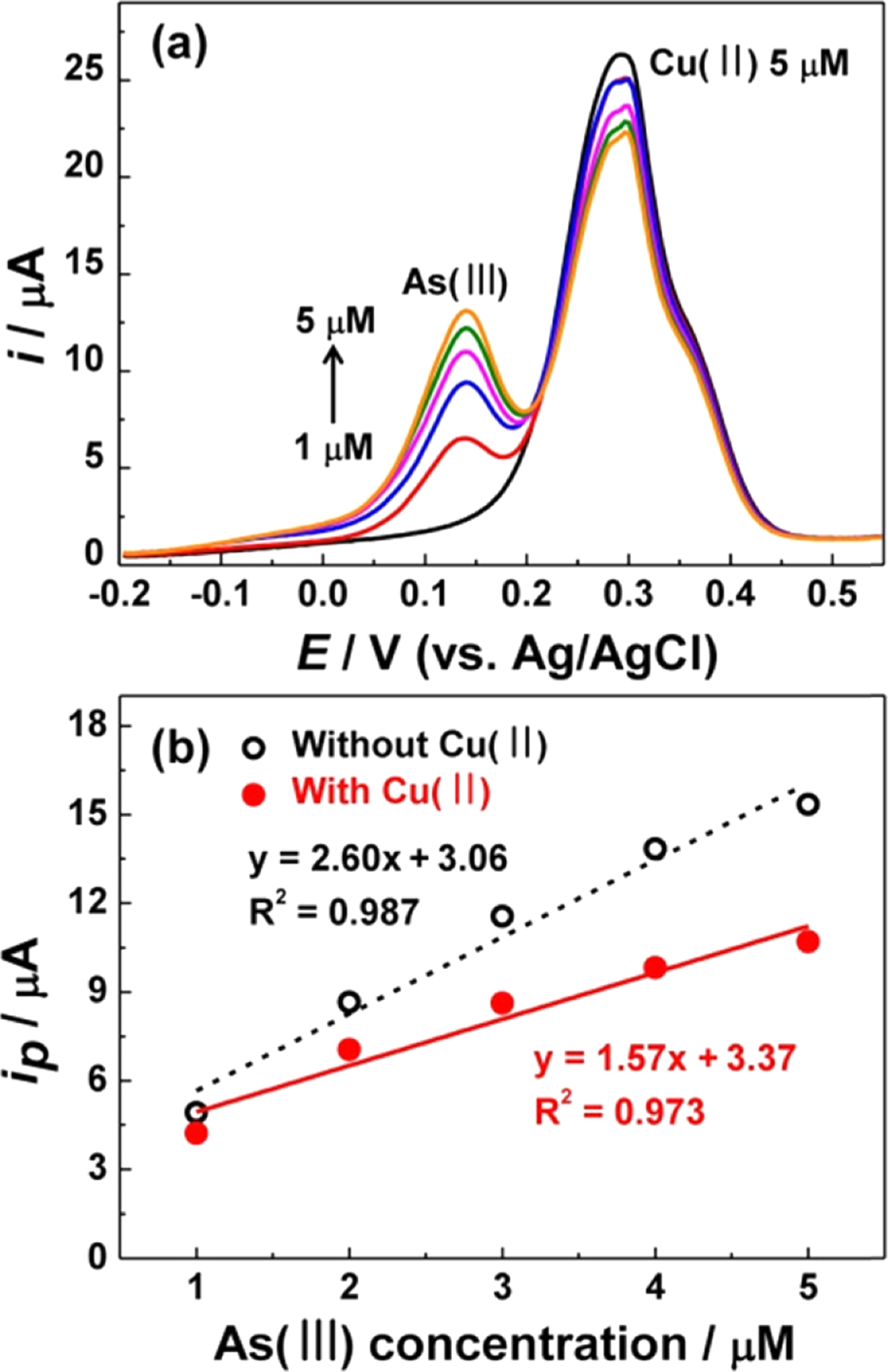
(a) SWVs obtained on flat Au electrodes in 1 M HCl containing various concentrations of As(III) in the presence of 5 μM Cu(II).
Deposition potential = -0.2 V and pre-deposition time = 150 s. (b) Calibration curves of As detection in the presence and
absence of Cu(II).
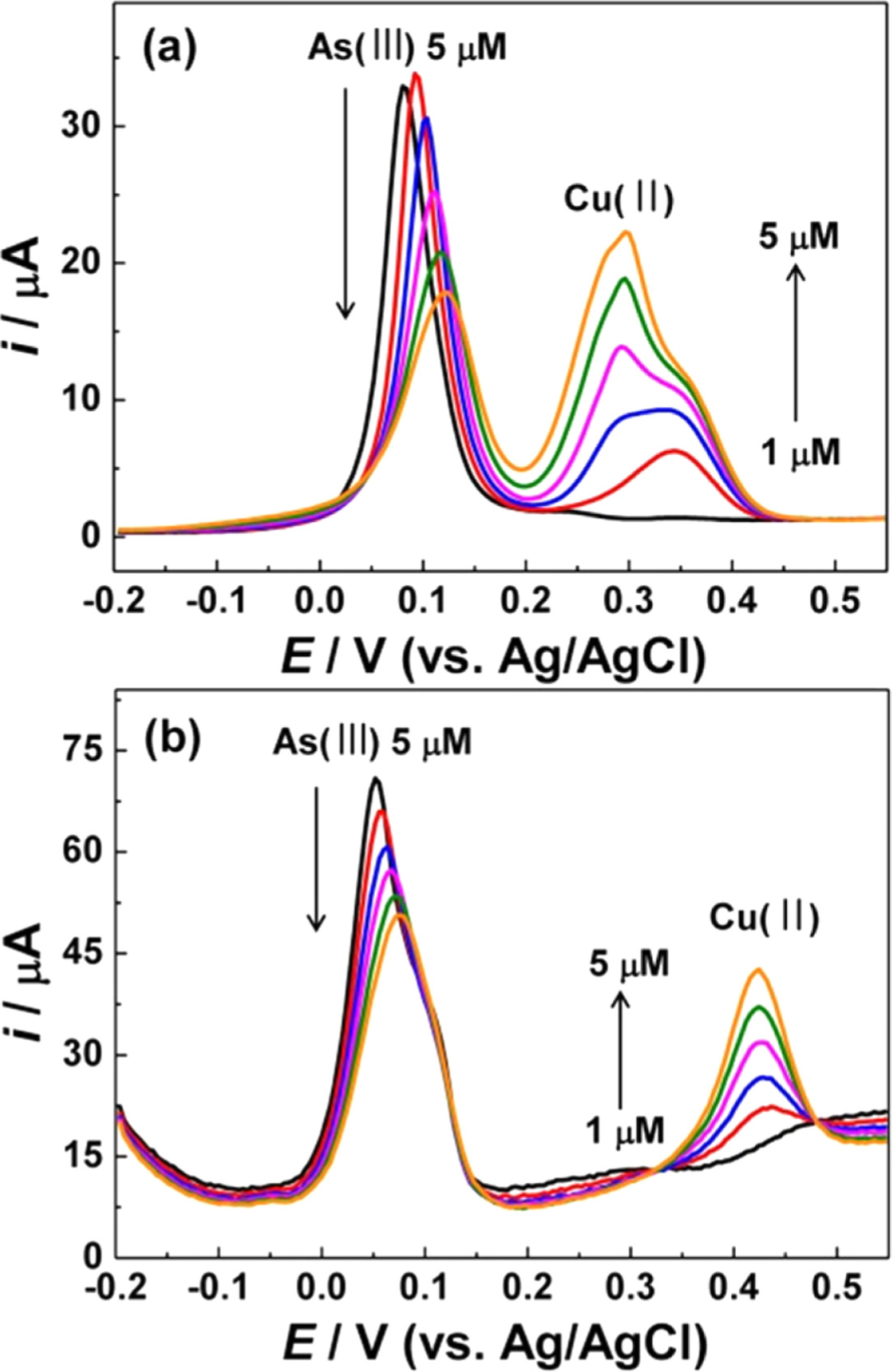
FigureS6.
SWVs obtained on (a) flat Au and (b) NPG (50 s) electrodes for 5 μM As(III) in the presence of 1 ~ 5 μM Cu(II) in 1.0 M HCl.
Deposition potential = −0.2 V and pre-deposition time = 150 s.