Journal Information
Journal ID (publisher-id): chemical
Title: Journal of the Korean Chemical Society
Translated Title (ko): 대한화학회지
ISSN (print): 1017-2548
ISSN (electronic): 2234-8530
Publisher: Korean Chemical Society대한화학회
The simple Grunwald-Winstein equation1 (eq. 1) and the extended Grunwald-Winstein equation2 (eq. 2) have been used to study the reaction pathways of the solvolysis reactions. The sensitivities (l and m) of the rates of solvolyses towards changes in solvent nucleophilicity (NT) and solvent ionizing power (YCl) are closely related to the relative amounts of bond making and bond breaking at the transition state (TS) of the rate-determining step.3
Based on the successful correlations of the rate constants of solvolyses of benzoyl chloride derivatives, it is concluded that the application of the extended Grunwald-Winstein equation (eq. 2) could provide valuable mechanistic information concerning the solvolyses reactions at an acylcarbon.4 In previous papers in this series, we have reported that solvent effects on the TS variation in the reaction of carbonyl derivatives (RCOCl or ROCOCl) in various mixed solvents.5 These solvolysis reaction can be shown as Scheme 1.
In this work, we investigated transition state variations in the solvolysis reactions of anthraquinone-2-carbonyl chloride (1) as equation 3. Anthraquinone-2-carbonyl chloride has been used as an electron-affinity alcohols derivative.6
The rate constants for the solvolyses of anthraquinone-2-carbonyl chloride (1) were obtained at 35.0 °C by the conductivity method and are reported in Table 1. The rates of reactions were relatively fast in strongly nucleophilic solvent (e.g., H2O) and were relatively slow in strongly electrophilic solvent in Table 1. The rate constants were more dependent on the solvent nucleophilicity than on the solvent ionization power.7
aPrepared on a volume/volume basis with other component water.
b Value of k (= 4.02×10-2s-1) in methanol-d (MeOD), corresponding to a kMeOH/kMeOD value of 2.27.
c Prepared on a weight/weight basis with other component water.
Typically, bimolecular reactions have been reported to have larger rate constants in nucleophilic solvent (H2O) systems and smaller ones in electrophilic solvent systems. Therefore, it can be suggested that the solvolysis of 1 is a bimolecular reaction mechanism.8
When all rate constants were analyzed by the simple Grunwald-Winstein equation (eq. 1),1 presented in Fig. 1 show significant scattered for the TFE-H2O and TFE-EtOH solvents. This result would expect normally the “push-pull” mechanism of SN2 reactions.9
The analysis using the extended Grunwald-Winstein equation2 showed an improved result (Fig. 2) which have 2.11 ± 0.11 for the l value and 0.54 ± 0.06 for the m value, 0.37 for the c value, with 0.955 as the correlation coefficient (R).
The extended Grunwald-Winstein equation is very significant for a mechanistic interpretation of solvolytic reactions. The nucleophile contribute to the reaction in the bimolecular reaction. On the contrary, in a single-molecule nucleophilic substitution reaction, the nucleophile cannot affect the rate constant.2
When the nucleophilic substitution reaction proceeds via the SN1 reaction, the l value is ≈ 0 and the m value is ≈ 1, whereas the l value is ≈ 1.0 and the m value is ≈ 0.50 in the SN2 reaction, and the l value is ≈ 1.5 and the m value is ≈ 0.60 in the associative SN2 (or addition-elimination) reaction.2
The l value of 2.11 is similar to those previously reported for associative SN2 reactions. Solvolyses of 4-nitrobenzoyl chloride10 (4-NO2C6H4COCl, l = 1.78), benzyl chloroformate8 (C6H5CH2OCOCl, l = 1.95), phenyl chloroformate5c (C6H5OCOCl, l = 1.68), and methyl chloroformate11 (CH3OCOCl, l = 1.59) in which a chloride ion is displaced from carbonyl carbon.
In the extended Grunwald-Winstein equation, the ratio of the l and m values (l/m) can be a useful indicator to predict the reaction mechanism.2,3 When the solvolysis proceeds via an associative SN2 reaction, the value of l/m is about 2 to 3. On the other hand, the value of l/m is less than 1, it can be expected that proceeds via a SN1 reaction (or an ionization reaction). The value of l/m is 3.9 within the range of values typically found in associative SN2 reaction in the solvolysis of 1. This result can be expected that the solvolysis of anthraquinone-2-carbonyl chloride (1) proceeds by associative SN2 reaction.2,3
A Brønsted-Lowry acid is a substance that donates a proton (hydrogen ion, H+) to another substance and a Brønsted-Lowry base is a substance that accepts a proton.12 The reaction rate in the general base catalytic reaction is proportional to the total amount of the base present. The solvolysis reaction of carbonyl compound derivatives are widely used as a subject for general base catalysis.13 In this study, it can be expected that reacting with weakly basic alcohols or carbocation of the carbonyl group can be stabilized by a catalyst to promote the reaction.14
The solvolyses of carbonyl derivative compounds proceeding with bimolecular reaction may undergo the transition state (TS1) as the general base catalysis reaction mentioned above. The contribution of the second methanol solvent may be closely related to the reaction rate in TS1 because the first molecule of methanol (a) affects the ability attacks the carbonyl carbon of the substrate in TS1. In the case of bimolecular (SN2) reactions, the force constant of the H-O(a) vibration modes will decrease and this will lead to a primary solvent kinetic isotope effect in TS1.5c,d,15 The SKIE (kMeOH /kMeOD) is 2.27 in the solvolysis of 1, which showed a relatively large primary solvent kinetic isotope effect. From these results, it can be expected that the solvolysis of 1 proceeds associative SN2 reaction pathway.15
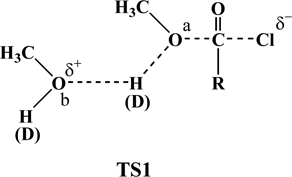
The overall situation for solvolysis of 1 which extended Grunwald-Winstein equation treatments of several kinetic studies involving displacement of chloride from carbonyl carbon, across the full range of solvents, strongly suggests the associative SN2 process with more bond formation than bond breaking at the transition state.2,3 This interpretation is further supported by a solvent kinetic isotope effect,16 kMeOH/kMeOD = 2.27.
The solvents were refined and used as described in previous papers. Anthraquinone-2-carbonyl chloride (1) was purchased. The reaction rate was measured by the conductivity method using a self-made tool.16 The rate constant is the average of the rate constants measured in two or more experiments and the uncertainty value is within ±3%.
[(a)] E. Grunwald S. Winstein J. Am. Chem. Soc.846701948 [(b)] S. Winstein E. Grunwald H. W. Jones J. Am. Chem. Soc.1951732700 [CrossRef]
[(a)] I. S. Koo T. W. Bentley D. H. Kang I. J. Lee Chem. Soc., Perkin Trans.19912296 [(b)] D. N. Kevill M. J. D’Souza J. Chem. Res. Synop.1993174 [(c)] J. B. Kyong B. C. Park C. B. Kim D. N. Kevill J. Org. Chem.2000658051 [CrossRef] [(d)] J. B. Kyong J. S. Yoo D. N. Kevill J. Org. Chem.2003683425 [CrossRef]
[(a)] D. N. Kevill M. J. D’Souza J. Chem. Soc., Perkin Trans.20022240 [(b)] J. B. Kyong J. Rhu, C Y. G. Kim D. N. Kevill J. Phys. Org. Chem.200720525 [CrossRef]
K. H. Park D. N. Kevill J. Phys. Org. Chem.2012252 [CrossRef]
[(a)] R. F. Hudson G. W. Loveday J. Chem. Soc.1966766766 [(b)] J. B. Hyne R. Will J. Am. Chem. Soc.1959812371 [CrossRef] [(c)] K. H. Yew H. J. Koh H. W. Lee I. Lee J. Chem. Soc., Perkin Trans.199522263 [(d)] D. N. Kevill M. J. D’Souza J. Chem. Soc., Perkin Trans.199721721
K. Naito A. Miura M. Azuma J. Am. Chem. Soc.19911136386 [CrossRef]
H. J. Koh S. J. Kang Bull. Korean Chem. Soc.2015362429 [CrossRef]
D. N. Kevill M. J. D’Souza J. Phys. Org. Chem.200215881 [CrossRef]
D. N. Kevill J. H. Ryu M. A. Neidermeyer F. Koyoshi M. J. D’Souza J. Phys. Org. Chem.200720431 [CrossRef]
I. Lee W. H. Lee H. W. Lee J. Phys. Org. Chem.19936361 [CrossRef]
X. G. Zhao S. C. Tucker D. G. Truhlar J. Am. Chem. Soc.1991113826 [CrossRef]
[(a)] H. J. Koh S. J. Kang D. N. Kevill Bull. Korean Chem. Soc.2008291927 [CrossRef] [(b)] I. Lee H. J. Koh Y. S. Park H. W. Lee J. Chem. Soc. Perkin Trans.199321575